Graduate Pharmacy Aptitude Test (GPAT) and Pharmaceutical Competitive Exams
Graduate Pharmacy Aptitude Test (GPAT)

Graduate Pharmacy Aptitude Test (GPAT), an all-India examination, was launched by the All India Council for Technical Education (AICTE), New Delhi, with effect from the academic year 2011–12 for admission into the Master's Programme in Pharmacy (M.Pharm) and for awarding fellowships.
The first GPAT examination (GPAT-2010), and the subsequent ones in 2011 and 2012, were conducted in paper-pencil mode by AICTE in association with M.S. University of Baroda, Vadodara.
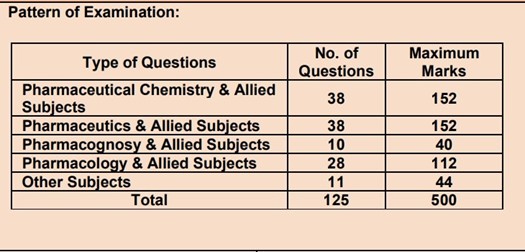
Paper Pattern
Highlights
- You will be taught by well-qualified and experienced teachers who care about your success. From basic concepts to advanced topics, everything will be explained in a simple and easy-to-understand way. Our trainers stay updated with the latest trends, often covering exam-relevant topics that are not usually found in routine textbooks — essential for exams like GPAT, NIPER, BITSHDPG, MANIPALCET, etc.
- We provide detailed and easy-to-understand notes that are as reliable as textbooks and much more concise and reader-friendly.
- Full-length material and an online test series (40 tests) will be provided to strengthen your preparation.
NIPER Coaching | NIPER Coaching Hyderabad

Clin Infotech is the first institute to offer dedicated NIPER coaching, established in the year 2001. It provides result-oriented, high-quality coaching for NIPER, guided by experienced faculty members.
Clin Infotech shapes your career path effectively, serving as a strong platform not only for GPAT and PGECET, but also for NIPER, Drug Inspector, Pharmacist, and other competitive examinations.
NIPER JOINT ENTRANCE EXAM (NIPER JEE 2014)
National Institute of Pharmaceutical Education and Research (NIPER) Ahmedabad; Guwahati; Hajipur; Hyderabad; Kolkata; Rai Barelli; S.A.S. Nagar
Medicinal chemistry
- IUPAC nomenclature, R and S nomenclature, E and Z isomerism.
- Conformations
- Hybridization, aromaticity, Huckles rule reaction mechanisms- Electrophilic, Nucleophilic , SN1, SN2, Elimination E1 E2 etc.
- Ester hydrolysis, Aac1 Aac2……all eight mechanisms(Jerry march) Markovnikoves rule, Bredts rule, Stereoselectivity, stereospecificity, regioselesctivity, chemoselectivity, chirality, stereochemistry, conformations, rearrangements, acids and bases.
Pharmacology and toxicology:
- Pharmacokinetics, pharmacodynamics, pharmacological effect, desired, undesired, toxic, adverse effects.
- Bioavailability, bioequivalence, various factors of ADME
- Drug metabolism: various pathways and other details.
- Drug interactions, agonist, antagonist, partial agonist, protein binding, drug distribution, distribution volume, excretion pathways etc.
- Mechanism of drug action, drug-receptor interaction.
- Various adrenergic, cholinergic and other receptors
- Detailed study of CNS pharmacology specially opiod receptors
- Study of basis of threshold areas of work in NIPER in pharmacology dept. mentioned in brochure.
Pharmaceutical analysis:
- Stability testing of pharmaceuticals, various stability tests, kinetic studies, shelf life determination, thermal stability, formulation stability.
- Various analytical techniques
- Tests: physical and chemical tests, limit tests, microbiological tests, biological tests, disintegration and dissolution tests.
- Thermal techniques: DSC, DTA, TGA, etc.
- Chromatography- detailed.
- QA and QC: GLP, TQM, ISO system.
Biotechnology
Here biotechnology is somewhat different than normal biotechnological institute so not too much worry about this portion.
- Gene expression, mutation, replication, transcription, translation, recombination, bacteriophages.
- Fermentation: fermenters, fermentation process, its regulation, conditions, bioprocessors, various enzymes in fermentation technology. Fermentation of Antibiotics, vitamins, amino acids, hydroxy acids such as lactic acid etc.
- Gene therapy: methods and applications.
- Monoclonal antibodies, insulin, interferon's, enkephylins, angiotensin, analogues and other peptides.
- Enzymes, types of enzymes and enzyme kinetics etc.
- Use of microorganisms in pharmaceutical industries.
Practice of Pharmacy:
- Adverse Drug Reactions,
- Rational drug use as well as some typical case studies in diabetes and hypertension and
- Some case study regarding cvs and anti-infective.
- Therapeutic drug monitoring
- Hospital pharmacy & Clinical research
- You should give attention to statistics in which mean, median, mode, anova, paired ttest.
- Pharmacy Act and D&C act. And knowledge about important laboratories of India and there location.
Post Graduate Engineering Common Entrance Test (PGECET)

Clin Infotech provides result-oriented, excellent coaching for PGECET by experienced faculty members. The institute guides you in shaping your career the right way and serves as a platform for GPAT, PGECET, NIPER, Drug Inspector, Pharmacist, and other competitive examinations.
Post Graduate Engineering Common Entrance Test (PGECET) is an AP State Level Common Entrance Test for admission into regular PG courses in Engineering, Technology, Architecture, and Pharmacy (ME/M.Tech./M.Pharmacy/M.Arch), and Graduate-level Pharm-D (Post Baccalaureate). It is conducted by the University College of Engineering, Osmania University on behalf of APSCHE — a statutory body of the Government of A.P., Hyderabad.
PGECET Syllabus for Pharmacy:
Pharmacognosy & Phytochemistry:
Chemistry of natural products, tests, isolation, purification &characterization and estimation of phytopharmaceuticals belonging to the group of Alkaloids, Glycosides, Terpenoids, Steroids, Bioflavanoids, Purines,lipids, proteins. Pharmacognosy of crude drugs and herbal products. Standardization of raw materials. Modern techniques used for evaluation.
Pharmaceutical Chemistry:
Structure, nomenclature, classification, synthesis, SAR and metabolism of the following category of drugs, which are official in Indian Pharmacopoeia and British Pharmacopoeia. Introduction to drug design. Stereochemistry of drug molecules. Hypnotics and Sedatives, Analgesics, NSAIDS, Neuroleptics, Antidepressants, Anxiolytics, Anticonvulsants, Antihistaminics, Local Anaesthetics, Cardio Vas- cular drugs – Antianginal agents Vasodilators, Adrenergic & Cholinergic drugs, Cardiotonic agents, Diuretics, Antijypertensive drugs, Hypoglycemic agents, Antilipedmic agents, Coagulants, Anticoagulants, Antlplatelet agents.Chemotherapeutic agents – Antibiotics, Antibacterials, Sulphadrugs. Antiproliozoal drugs, Antiviral, Antituber- cular, Antimalarial, Anticancer, Antiamoebic drugs. Diagnostic agents.
Pharmaceutics:
Formulation, Development and Storage of different dosage forms and new drug delivery systems. Biopharmaceutics and Pharmacokinetics and their importance in Pharmaceutical calculations. Study of physical properties of drugs: Particle size and shape, pKa, solubility, partition coefficient, crystallinity, polymor- phism and hygroscopicity. Study of chemical properties of drugs: Hydrolysis, oxidation, reduction, recimization, polymerization and their influence on formulation and stability of drug products.
Pharmacology:
General pharmacological principles including Toxicology. Drug interaction and Pharmacology of drugs acting on Central nervous system, Cardiovascular system, Autonomic nervous system, Gastro intestinal system and Respiratory system. Pharmacology of Autocoids, chemotherapeutic agents including anticancer drugs, Bioassays, Immuno Pharmacology. Drugs acting on the blood & blood forming organs. Clinical Pharmacy Therapeutic Drug Monitoring Dosage regimen in Renal and hepatitic impairment. Drug – Drug interactions and Drug -food interactions, Adverse Drug reactions. Medication History, interview and Patient counseling
Pharmaceutical Analysis and quality assurance:
Concepts of qualitative and quantitative analysis, fundamentals of volumetric analysis, methods-of expressing concentration, primary and secondary standards; concept of error, precision, accuracy, specificity, sensitivity, detection limit, linearity and range. Ruggedness, standards, standardization, calibration of analytical equipments. Principles, instrumentation and applications of the following: Absorption spectroscopy (UV,visible & IR ) .Fluorimetry, Fla me photometry, Potentiometry.Conductometry and Plarography. Pharmacopoeial assays and chromatography methods.Quality assurance and quality control methods, concepts of GMP and GLP and forensic pharmacy.
Pharmaceutical Biotechnology:
Isolation, classification and taxonomy of microorganisms. Pure culture techniques, theory and practice of sterilization, microbial growth phases and kinetics, microbial transformation of steroids. Fermentation technology- batch and continuous fermentation. General characteristics and manufacture of antibiotics, vaccines and harmones. Application and scope of recombinant DNA technology in manufacture of biological products such as insuline and human growth harmones. Biochemical role of hormones, Vitamins, Enzymes, Nucleic acids, Metobolic path.ways-glycolosis and TCA cycle and transport across cell membranes.
Highlights:
- You will be taught by well qualified and experienced teachers who care about you. Basic concepts to advanced level will be taught in a simple and easy way to understand the concepts. They know the current trends and advancement in their fields and often update the students of the facts which they themselves cannot know from routine books and often such questions are asked in examinations like GPAT, NIPER, BITSHDPG, MANIPALCET, etc.
- We provide detailed and easy to understand notes. These are as good as your text books in terms of authenticity and are extremely concise and easy to read
- We will provide the full length material & online test series (40 online tests)
Drug Inspector

Clin Infotech is the first institute to offer dedicated Drug Inspector coaching, established in the year 2001. The institute provides result-oriented, high-quality coaching for Drug Inspector exams with guidance from experienced faculty members.
Clin Infotech helps you shape your career path effectively and is also a trusted platform for GPAT, PGECET, NIPER, Pharmacist, and other competitive examinations.
PLAN OF DRUG INSPECTOR EXAM
1. The written examination will consist of two papers with 250 marks.
| Paper | Subject | Maximum Marks | No. of objective multiple choice question | Duration of the Examination |
|---|---|---|---|---|
| Paper-I | Pharmacy | 200 | 100 | 2 Hours |
| Paper-II | General Knowledge | 50 | 50 | 1 Hour |
2.
- All question papers will be set in English and the same should be answered in English only.
- The candidates are not allowed to bring calculators or any other electronic devices to the examinationhall/examination campus for use.
- Mobile phones, pagers or any other communication devices are not allowed inside the premises of theExamination Centre and Office of the Commission.
Any infringement of these instructions shall entail disciplinary action including ban from future examination.
3. will be negative marking for wrong answers.
SYLLABUS FOR WRITTEN EXAMINATION FOR RECRUITMENT TO THE POST OF DRUGS INSPECTOR.
PAPER-I PHARMACY
There should be 8 units containing the following:
Unit-1- FORENSIC PHARMACY
- Drugs and Cosmetic Act, 1940 and Rules thereunder, 1945 with amendments.
- Pharmacy Act, 1948.
- Drug Price Control Order, 1995.
- Medical Termination of Pregnancy Act, 1971.
- Poison Act, 1919 and Dangerous Drugs Act, 1930.
- Drugs and Magic Remedy Act, 1954.
- Medical and Toilet Preparation Act, 1955.
- Prevention of Cruelty to Animal Act.
- Trademark Registration Act.
- Pharmaceutical Ethics.
Unit-2- MANUFACTURING PHARMACY
- Tablet and Tablet coating.
- Capsule.
- Emulsion, Suspension, Ointment and Cream.
- Ophthalmic Solutions.
- Blood Fluid and Electrolytes.
- Parenteral preparation and Quality Control.
- Surgical Dressing.
- Biological preparation … (Sera, Vaccine and Anti-Sera)
- Biopharmaceutics.
Unit-3- PHARMACEUTICAL ANALYSIS
- Limit Test.
- Bio-Assay.
- Sterility Test.
- Pyrogen Test.
- Theory & Application of Colorimeter, Florimeter, Nephlometer and Turbidometer, U.V.Visilile Spectrophotometer.
- Kerl Fischer Titration.
- Alcohol determination.
- Microbiological Assay of Vitamins, Antibiotics and Vaccine Preparation.
Unit-4- MEDICINAL CHEMISTRY
Structure, Storage, Preparation & Brand names of the Following Classes (Definition, Classification etc.):
- Steroids
- Sedatives and Hypnotics.
- Psycho-therapeutic Agents.
- Antihistaminic Agents.
- Analgesics (narcotic, non-narcotic and NSAID)
- Cardiovascular Agents.
Unit-5 -PHARMACOGNOSY
Source, Chemical constituents, uses and adulteration of the following classes of natural drugs, Rauwolfia, Ipecacuahna, Belladona, Cinchona, Cinnamon, Digitalis, Senna, Aloe, Noxvomica, Opium, Kurchi, Brahmi, Tulsi, Bael and Ephedra.
Unit-6- PHARMACOLOGY & TOXICOLOGY
Introduction and General Principle
Mode of action, Drug receptor interaction, Drug, antagonist, Absorption, distribution, metabolism and excretion of drugs, Routes of administration, Bioavailability, Drug dependence and addiction, Drug abuse and toxicity, Adverse drug reaction, Drug allergy, Biostatistics.
Unit-7- HOSPITAL & CLINICAL PHARMACY
Handling of prescription, Incompatibility, Storage conditions of drugs, Clinical Pharmacy and its role in Hospital.
Unit-8 - ANATOMY, PHYSIOLOGY & HEALTH EDUCATION
- Elementary knowledge of following systems :- Blood, Digestive system, Respiratory system, Eye, Ear, Reproductive system and Urinary system.
- Nutrition, First aid, Population Control, Aids Control.
PAPER-II (GENERAL KNOWLEDGE):
The paper in General Knowledge will include knowledge of current events and matters as of everyday observation and experience in their scientific aspects of life as may be expected of an educated person. The paper will also include questions on History of India and Geography of such standard which the candidates should be able to answer without special study.
| Paper | Subject | Maximum Marks | No. of objective multiple choice question | Duration of the Examination |
|---|---|---|---|---|
| Paper-I | Pharmacy | 200 | 100 | 2 Hours |
| Paper-II | General Knowledge | 50 | 50 | 1 Hour |
Clin Infotech ADVANTAGE: Why Clin Infotech For Drug Inspector Coaching
- Exclusive faculty of pharma-experienced profiles from reputed organizations.
- Erudite & eminent faculty at national and international level.
- Fixed number of coaching hours delivered on time.
- Full material with exercises and solutions for practice.
- Practice test series aligned with exam pattern and workshops with faculty.
- Free career counselling and job guidance to help excel in career.
- Library facility and well-maintained ambience at the office.
- Convenient batch timings for students and working professionals.

For more information, please contact Clin Infotech at:
Email: careers@clininfotech.com
Phone: 8374457387
Additional Resources
- GPAT Exam Questions
- NIPER Exam Questions
- PGCET Exam Questions
- Drug Inspector Questions


 Get Brochure to Your Mail
Get Brochure to Your Mail Get Brochure to Your Mail
Get Brochure to Your Mail